High-Purity Buspirone Impurity Standards for Pharmaceutical Use
High-Purity Buspirone Impurity Standards for Pharmaceutical Use
Blog Article
Buspirone impurities can form during the synthesis of the active pharmaceutical ingredient (API) and formulation processes due to side reactions, degradation of the compound under specific environmental or processing conditions, or contamination from solvents and reagents. These impurities may result from unstable intermediates, over-reaction, or deviations in reaction parameters during synthesis.

Buspirone Impurity A
CAS No.: 20980-22-7

Buspirone Impurity B
CAS No.: 81461-73-6

Buspirone Impurity C
CAS No.: 257877-45-5

Buspirone Impurity E
CAS No.: 257877-43-3

Buspirone Impurity F
CAS No.: 2512210-24-9

Buspirone Impurity G
CAS No.: 84746-24-7

Buspirone Impurity I
CAS No.: 2725354-99-2
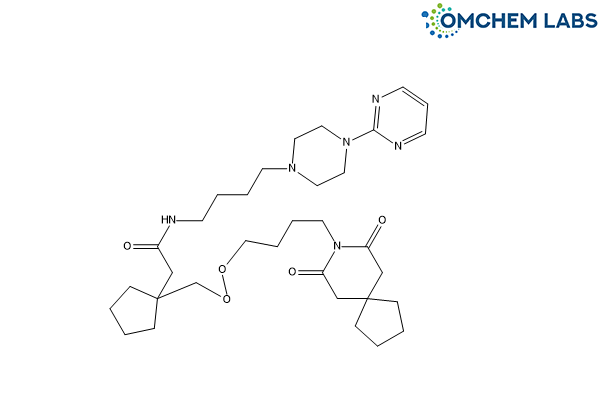
Buspirone Impurity J
CAS No.: 2726492-72-2

Buspirone Impurity K
CAS No.: 1075-89-4

Buspirone Impurity L
CAS No.: 21098-11-3

Buspirone Impurity M
CAS No.: 80827-62-9

Buspirone Impurity N
CAS No.: 257877-44-4

Buspirone Hydrochloride
CAS No.: 33386-08-2
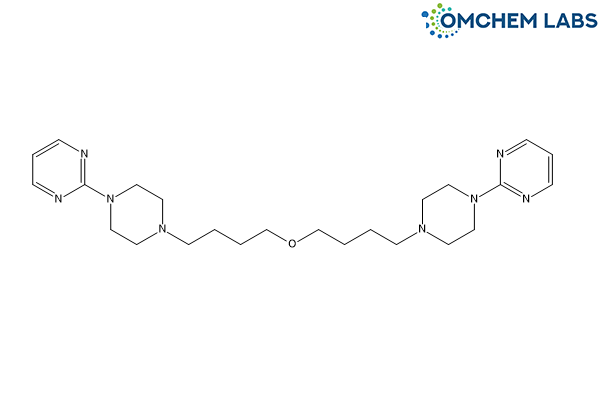
Buspirone Impurity D (Free Base)
CAS No.: 2724726-67-2

Buspirone Impurity D (HCL)
CAS No.: N/A

Buspirone N-Oxide
CAS No.: 220747-81-9